There has never been a drug, which is still under clinical trials and only one clinical report, that prompted so many countries racing to replicate and produce the treatment.
Remdesivir, made by American biopharmaceutical company Gilead Sciences, was seen as the antiviral hope against COVID-19 after The New England Journal of Medicine published a preliminary report on the drug’s immediate effect on a coronavirus patient.
Not only did China started conducting clinical trials and replicating the drug on February 20, two of Taiwan’s most prestigious research institutes — Academia Sinica and National Health Research Institute (NHRI) — also announced on the same day that they have completed the synthesis of Remdesivir with 97-percent purity.
Fighting a global public health crisis is an all-out war. The real purpose behind such “talent display” is not aimed at grabbing the business opportunity to produce the antiviral drug, but at “flexing muscles.”
“We have the ability to synthesize this drug, but we hope it won’t be ‘deployed’ in the end,”said Chen Chiung-Tung (陳炯東), director of Biotechnology and Pharmaceutical Research at NHRI.
Not deploying Remdesivir might represent one of these two scenarios: either COVID-19 has been contained, or Gilead Sciences has supplied Taiwan with enough Remdesivir treatment in times of urgent need.

To accomplish the mission of developing a drug that “hopefully will not be used,” research teams from Academia Sinica and NHRI started working day and night since the beginning of February for the synthesis of Remdesivir.
At the time of The Reporter’s visit, NHRI’s Biotechnology and Pharmaceutical Research team had completed 80 percent of the synthesis operations, reaching the final stage of crystallization and scale-up. The research teams had put aside their original projects to make a run for the Remdesivir replication, even considering putting a bed in the lab for 24-hour operations.
Meanwhile, the young and diverse team led by Chein Rong-Jie (陳榮傑), associate researcher at Academia Sinicia’s Institute of Chemistry, was catching up. While the NHRI, a “mission-driven” institute that had assisted in synthesizing Tamiflu, an antiviral drug used for the H5N1 avian influenza in 2005, Academia Sinica is the leader of Taiwan’s fundamental research. The team led by Chen had experiences in organic synthesis and cancer drug development, but the Remdesivir project was the first “national emergency mission” for them.
“When people ask us about the difference between NHRI and Academia Sinica or schools, I think it’s clear: when our country is in crisis, we have the duty to stop our current work to assist the government. Otherwise we normally conduct research that might be helpful for government policy; we play the role of a think tank,” said Liang Keng-Yee (梁賡義), Director of the NHRI.
Scientists play an ever-important role in the global COVID-19 crisis. National Tsing Hua University (NTHU) professor Lee Chia-Wei (李家維) urged Taiwanese scientists to work together on February 4. James C. Liao (廖俊智), President of Academia Sinica, gathered talent from the Ministry of Health and Welfare, National Biotechnology Research Park, Development Center for Biology, the NHRI, and the private sector to form a cross-platform “national team.” While a concrete science platform was not established, every ecosystem was working towards the same goal with its own resources and energy.
“In terms of drug synthesis, having multiple teams to work simultaneously reduces the risks of development,” Chein said.
Aside from Academia Sinica and the NHRI, other research units in the country were also working on the synthesis. NHRI’s Biotechnology and Pharmaceutical Research director Chen Chiung-Tung(陳炯東) said it was good to have “positive competition” and the researchers “knew that everyone was working hard on the synthesization.”
In the end, Academia Sinica spent 14 days, while the NHRI spent 16 days, on completing the Remdesivir synthesis at a 97-percent purity level.
What were the technical challenges and keys to replicating Remdesivir within 14 days?
Both Chen and Chein think new drug development is the hardest as it is time consuming to go from zero to having something substantial. Synthesizing a drug that has already been made, however, has less of a technical barrier.
“But the structure of this drug is relatively complicated,” Chein added.
Remdesivir’s original producer Gilead Sciences had already published the major steps to synthesize the drug in a medical journal in 2017. The whole world basically knows how to synthesize this particular drug.
“We ordered three to four of the required raw materials two weeks in advance, but we still couldn’t get one of them. We had to synthesize even the raw materials, so the original chemical steps increased from 11 to 20; it was more time consuming,” Chein said.
NHRI’s Biotechnology and Pharmaceutical Institute assistant researcher Chang Chun-Ping (張竣評) said as soon as the team received the request on February 2, they started cracking the chemical process and preparing the raw materials. He also discussed strategies with research Shia Ke-Shan (夏克山) , who found the treatment for H5N1 within 18 days back then, and associate researcher Lee Ching-Chi (李靜琪).
They started the synthesis process on February 5. “Perhaps every country had an urgent need for the raw materials so it was extremely hard to find them. Even the companies that specialize in producing the raw materials would need at least two weeks to deliver our custom order. Under time restraint, we could only synthesize the materials on our own,” Chang said.
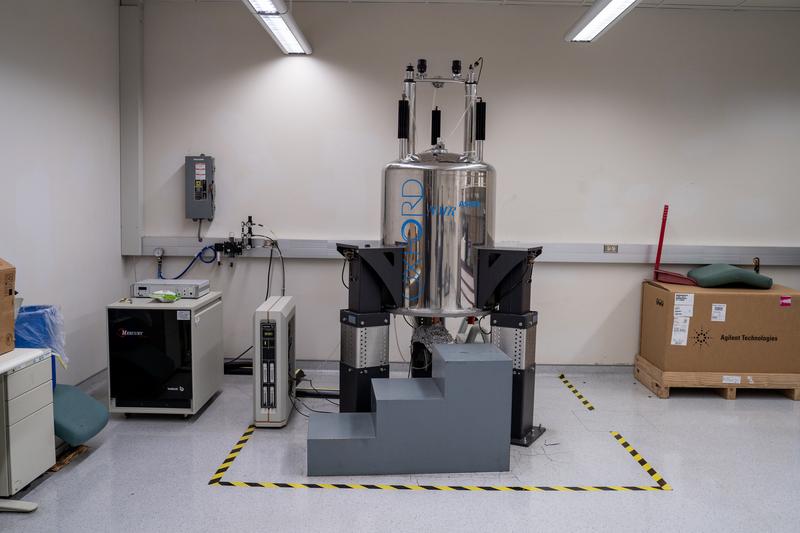
Drug thesis requires not only labor resources, but also nuclear magnetic resonance (NMR) spectroscopy. NHRI's Associate Director Ssutu Kang-Hui (司徒惠康) explained that drugs are made with small molecules with a very stable and precise structure of cyclocarbon, carboxyl, oxygen. MNR must be used to screen the compound structure to determine whether the synthesized drug is the same as the original and examine its purity level. As long as the overall structure is replicated with consistent quality, the purity level would also be sufficient.
An NMR spectrometer costs millions of dollars and it’s a core equipment for drug synthesis. The higher the magnetic field strength, the better the resolution. In the Academia Sinica’s Institute of Chemistry alone, there are six 400 and 500 MHz NMR spectrometers.
The two research teams at the NHRI were using 400MHz and 600MHz NMR spectrometers which were borrowed from Academia Sinica. “We’re not worried about overworking, but more so about the machines being broken,” Chen said in his report to the NHRI director Liang Keng-Yee (梁賡義). Liang made a swift decision to increase the budget to purchase extra NMR equipment.
“The more difficult parts are confirming the path for synthesis and recrystallization. Once we overcome those challenges, we can scale up the production,” Chen said, adding that the NHRI estimated the scale-up process to take place in early March.

Remdesivir has yet to enter the market, but it has already become the hottest drug in the world — this was a pure coincidence.
NHRI’s Biopharmaceutical Sciences researcher Hsu Tsu-An (徐祖安) explained that the Remdesivir is used to inhibit RNA replicase, reducing the replication of the virus. “It was developed to treat the Ebola virus, but its drug effect did not have the best performance among the four drug candidates,” Hsu said.
Remdivisir was later discovered to be useful in suppressing SARS and MERS during animal testing. Since COVID-19, SARS, and MERS are all coronaviruses, American researchers thought of using the drug to treat COVID-19; and Remdesivir made a surprising comeback.
“In fact, Gilead Sciences developed Remdesivir out of fulfilling its ‘social responsibilities’ demanded by the international community. Since Ebola was raging in the poverty-stricken region of West Africa, where the poor economy and the market provided zero incentive for pharmaceutical companies,” Hsu said.
“Gilead’s drug did not perform as expected, but it is now the hottest celebrity drug candidate. This shows the efforts in research and development may not have been wasted, and they might still yield unexpected results,” Hsu said.
However, brand name medicine normally holds 20 years of patent protection. Never in history was there a drug replicated by different countries while it was still in clinical trials.
China-based BrightGene Bio-Medical Technology Co. announced on February 11 that it has successfully replicated the synthesis and manufacturing technology for Remdesivir. But the company emphasized that it would only “donate” to patients during the pandemic, sparking controversies over the drug’s patent right. Meanwhile, Gilead, the original maker of Remdesivir, started conducting a double-blind study on 761 coronavirus patients at the Wuhan Jinyintan Hospital.
Remdesivir was first invented by a team under the leadership of Yang Tai-Yin (楊台瑩) , a Taiwanese scientist at Gilead. The drug itself has already been granted patent in the United States, Japan, Australia, among other countries, until October, 2035. Despite the patent protection, Remdesivir has yet to be granted a drug permit anywhere other than the US.
“This is a gray area. The patent right to a drug does not protect the clinical trial stage, but only the product itself. In other words, you could put this drug through clinical experiments, but you can’t apply for a drug permit or sell it. This is why the Chinese biotech company said the drug was only for ‘donation,’” said Liao Chi-Chou (廖繼洲), intellectual property right committee at the Academia Sinica and former director of Taiwan’s Food and Drug Administration.

Inside the chemical synthesis lab at the NHRI, a fume cupboard is labeled “the origin of Taiwan’s Tamiflu” — that’s the glorious footprint of the NHRI research team.
In 2005, the H5N1 novel influenza outbreak stormed through society like the COVID-19 pandemic today — no one possessed the antibodies against H5N1. Before a vaccine was developed, the generic antiviral drug Tamiflu was seen as the hope for treatment. However, the drug manufacturer Roche heavily restricted production, causing countries around the world to purchase the drug in a panic.
Taiwan’s NHRI synthesized the drug in 18 days, showing that “Taiwan has the manufacturing capabilities.” It became a strong leverage in Taiwan’s negotiation with Roche, and Taiwan became the first country to issue a compulsory license for the generic production of Tamiflu.
Liao was one of the government officials representing Taiwan at the time. “It was truly a moment of pride in my life. It was the second case for Taiwan, and the first time for biopharmaceuticals to issue a compulsory license,” he said.
Tamiflu’s patent also belonged to Gilead, which was developing Remdesivir at the time, and Roche was the pharmaceutical manufacturer that earned the production rights. Taiwan then had to face two pharmaceutical companies in negotiation and litigation at the same time.
“It was a very stressful time. The two international pharmaceutical manufacturers hired four big name attorneys, whereas our health ministry only had one lawyer, Jennifer Lin (林秋琴). We had to win,” Liao said.
The key to Taiwan’s compulsory license win was the argument that “Taiwan might not manufacture the drug unless Roche does not provide enough supplies,” Liao explained. After the NHRI completed the synthesis process, three of Taiwan’s local pharmaceutical manufacturers produced enough Tamiflu supplies for a population of 100 million.
“Eventually we forced Roche to supply Taiwan with the drug. But the H5N1 outbreak was not as bad as we had imagined, so our locally produced Tamiflu was not deployed,” Liao said.
However, Liao told a story about how the local drug manufacturers that used to be rivals “became good friends” after the Tamiflu mission. After the Tamiflu patent expired, the experience from that year was a good practice for Taiwan’s manufacturing of generic drugs.
This is why both Academia Sinica and the NHRI emphasized that the synthesis of Remdesivir is only reserved for emergency usage. Like the case of Tamiflu, the race to synthesize Remdesivir was a show of our “armament strength” as well as a mental comfort for Taiwanese.
Although, National Taiwan University (NTU)’s Vice Principal Chang Shang-Chun (張上淳) said the NTU hospital has already reached an agreement with Gilead that the manufacturer will provide the drug for free when Taiwan needs it. It will be provided on a case application basis, and it will be applied and used in a compassionate-use program.
“But if the outbreak is out of control, and the demand increases, Gilead might not be able to meet the production demands. That’s why I’m insisting that this is not for manufacturing, but for reserved use,” Liang Keng-Yee said. “We’ve finished everything on the front end, completed the steps, and earned the experience. We had a drill on everything from raw materials, synthesis, to drug structure. Once there’s a need, we can hand it to a drug manufacturer for scale-up production.”
Ssutu described drug synthesis as “a leverage for future negotiations.” If there comes a time when the Taiwanese government needs to negotiate with Gilead, we can be shield protection. If necessary, we can use our manufacturing capabilities as a bargaining chip if Gilead refuses to supply us with the drug.

While countries around the world are aggressively manufacturing and storing Remdesivir, there is only one official report on using this drug as treatment of COVID-19.
The first confirmed case of coronavirus in the United States was a 35-year-old man who returned to the U.S. after visiting his family in Wuhan. On his fifth day of hospitalization, his chest radiograph showed evidence of pneumonia. On the seventh night, the doctor prescribed Remdesivir on a compassionate use basis. The next day, the patient no longer needed supplemental oxygen, and his level of oxygen saturation returned to around 94 to 96 percent, a shocking result. The case report was published in the New England Medical Journal on January 31; Remdesivir rose to fame and Gilead stock prices surged.
“A patient with severe symptoms used this drug and had obvious results within a day. It attracted the world’s attention, but still, it was only one case. China is now conducting a large-scale experiment with over 700 people, and that data is all the more important. However, we still don’t have any information currently so it’s hard to reach a conclusion,” Ssutu said.
Under the circumstances of a public health crisis, some researchers would publish a report on a case’s progress for global reference. Hence, the U.S. published a report based on only one clinical case. China has been conducting clinical trials since February 5 but remained quiet for at least half a month, according to Liao.
Based on Taiwan’s coronavirus cases, the recovered patients were only treated with antibiotics and Tamiflu, whereas Thailand even used HIV drugs to treat the virus. Fang Chi-Tai (方啟泰), professor at the NTU’s College of Public Health, explained that those who use HIV or Ebola treatment on coronavirus don’t have that many antiviral drug options. It takes far too much time to develop a new drug, Fang said, and doctors have to resort to what’s currently available. The drug effects, however, need to be proven with a lot more experiments.
Dr. Huang Yu-Cheng (黃玉成), physician at the Chang Gung Hospital’s pediatric infections department, questioned the early usage of Remdesivir in the U.S. case. “Was it really working because of the drug? Or did the patient recover naturally? Even in clinical trials with a group in experiment and a control group, a lot of patients recover just from receiving placebo. So, it’s too early to say whether the drug is effective,” Huang said.
However, if the unofficial drug is necessary for a severe case, Liao said that “it must be treated as a clinical trial usage, and the patient has to sign an authorization. Since the drug effects are unclear, the physician has to pay extra caution to the prescription. If there are serious side effects, whether to continue or to stop the drug usage has to be decided with the patient’s biggest benefits in mind.”
(Updated 2020.3.4)
Seen as the hope for treating COVID-19, Remdesivir has been synthesized by domestic research teams and biotech companies. They have also continued to scale up and increase the purity level in preparation for the potential manufacturing need.
On February 20, the NHRI and Academia Sinica respectively announced the successful replication of one milligram and 100 milligrams of Remdesivir at a 97-percent purity level. Four days later, the NHRI managed to synthesize 1 gram of Remdesivir and said that local manufacturers have already expressed the will to carry out mass production.
On February 26, the Academia Sinica team also announced to have synthesized 1 kilogram of the drug at a 99-percent purity level, completing the mission at hand. The same team will continue to research on revamping old drugs as well as new antiviral drugs, hoping to develop more medical treatment for COVID-19.
(To read the Chinese version of this article, please click: 不只是製藥、這是戰爭──中研院、國衛院搶做抗武漢肺炎新藥「瑞德西韋」真正目的)
深度求真 眾聲同行
獨立的精神,是自由思想的條件。獨立的媒體,才能守護公共領域,讓自由的討論和真相浮現。
在艱困的媒體環境,《報導者》堅持以非營利組織的模式投入公共領域的調查與深度報導。我們透過讀者的贊助支持來營運,不仰賴商業廣告置入,在獨立自主的前提下,穿梭在各項重要公共議題中。
今年是《報導者》成立十週年,請支持我們持續追蹤國內外新聞事件的真相,度過下一個十年的挑戰。